Abstract
Introduction: The "7+3" regimen, consisting of infusional cytarabine and an anthracycline, has been the mainstay of induction therapy for acute myeloid leukemia (AML) for four decades. With this treatment, complete remission (CR) is achieved in 50-75% of patients (Fernandez 2009). An alternative induction regimen, AcDVP16, is based on the principle of timed sequential therapy (Burke 1977), whereby initial chemotherapy serves to align any residual leukemia cells in S-phase. A subsequent pulse of chemotherapy is then administered for maximum cell kill. AcDVP16 consists of 45 mg/m2/day of daunorubicin and 667 mg/m2/day of infusional cytarabine administered on days 1-3, followed by 400 mg/m2/day of etoposide on days 8-10. As configured, AcDVP16 is an anthracycline-sparing regimen that also obviates the need for a day 14 bone marrow examination. Moreover, with myriad anthracycline and cytarabine dosing options, there is arguably no standard 7+3 regimen currently in use. While AcDVP16 offers the advantage of simplicity, little data exists on outcomes with this regimen. The purpose of the present study is to assess the efficacy and safety of AcDVP16 in adult patients with AML.
Methods: We retrospectively analyzed the charts of all newly diagnosed adult AML patients (age ≥ 18 years) treated with AcDVP16 at our institution between August 2013 and April 2017. Responses were defined as follows: CR, CR with incomplete platelet recovery (CRp), CR with incomplete hematologic recovery (CRi), or refractory. Composite CR (CRc) was defined as the aggregate of CR, CRp, and CRi. Survival outcomes were analyzed using the Kaplan-Meier method. Individual predictive factors (age, cytoreduction, and prognostic category) were compared between CRc groups (yes vs. no) using Fisher's exact test. Descriptive statistics were used for categorical and continuous variables.
Results: Forty-seven patients received AcDVP16 as initial induction therapy for AML. Ages ranged from 22-65 (median 45) years. By NCCN risk status, 17 patients (36%) were favorable risk, 7 (15%) intermediate risk, and 23 (49%) poor risk. Twelve patients (25.5%) were positive for a FLT3/ITD mutation, and 22 (46.8%) had a NPM1 mutation. Cytoreduction with cyclophosphamide and/or hydroxyurea was administered to 39% of patients prior to initiation of AcDVP16. Of 45 evaluable patients, CRc rate was 89% (CR 74%, CRp 4%, CRi 6%). The CRc rate was not clearly affected by prognostic category (p=0.13), age (p=0.99), or use of cytoreduction (p=0.38).
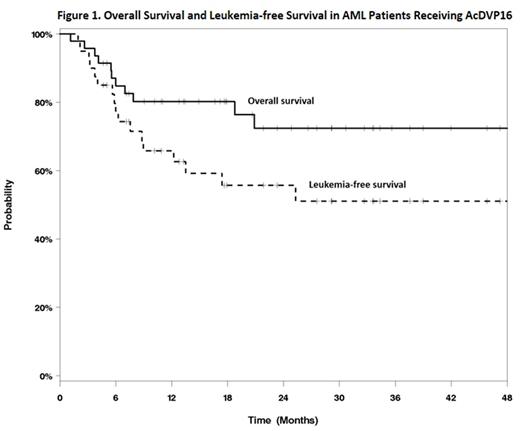
Among the entire cohort, one-year overall survival (OS) was 80.2%, and one-year leukemia-free survival (LFS) was 65.8%. Median OS and LFS were not reached during the study period (Figure 1). Of the 40 patients who achieved CRc, 37.5% relapsed after a median of 6.2 (range 1.9-25.2) months. Of 44 patients who recovered neutrophils following induction, recovery occurred at a median of 35 (range 23-77) days. Median inpatient length of stay was 36 (range 12-63) days. Grade 3/4 non-hematologic adverse events during induction consisted of the following: 7 (14.9%) dermatologic toxicity, 9 (19.2%) mucositis, and 9 (19.2%) hepatotoxicity. Six patients (12.8%) experienced cardiac toxicity, none with grade 3/4. All patients developed febrile neutropenia at some point during hospitalization. Of these, 46.8% had documented bacterial infection, 10.6% viral infection, and 8.5% fungal infection. There was 1 (2.1%) death during the induction hospitalization. Nineteen patients (40.4%) underwent allogeneic transplantation following AcDVP16: 5 (26.3%) with intermediate risk AML and 14 (73.6%) with poor risk disease.
Conclusions: This is a single arm cohort analysis examining the efficacy and safety of AcDVP16 induction therapy for AML. AcDVP16 provides an anthracycline-sparing alternative to 7+3 that yields a high survival rate, with a CR rate comparable to the best rates reported for 7+3. Although the time to neutrophil recovery is prolonged with this regimen, the risks are acceptable. Additionally, AcDVP16 avoids the controversy associated with the management of persistent bone marrow disease at day 14 post induction. These findings warrant validation in a larger prospective cohort.
Grunwald: Incyte: Consultancy, Research Funding; Alexion: Consultancy; ARIAD: Consultancy; Pfizer: Consultancy; Celgene: Consultancy; Forma Therapeutics: Research Funding; Genentech: Research Funding; Amgen: Consultancy, Research Funding; Cardinal Health: Consultancy; Janssen: Research Funding. Trivedi: Pharmacyclics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal